
Introducing: EnSite™ X Software Version 3
We create bold solutions to challenge AFib. You rely on technology bold enough not to compromise between efficiency and accuracy. EnSite X Software Version 3 introduces features that allow you to elevate your practice and meet the unique challenges of each case.


Workflow Optimization with EnSite X Software Version 3
EnSite™ Aid Module
Increase confidence while ablating with AutoMark distances
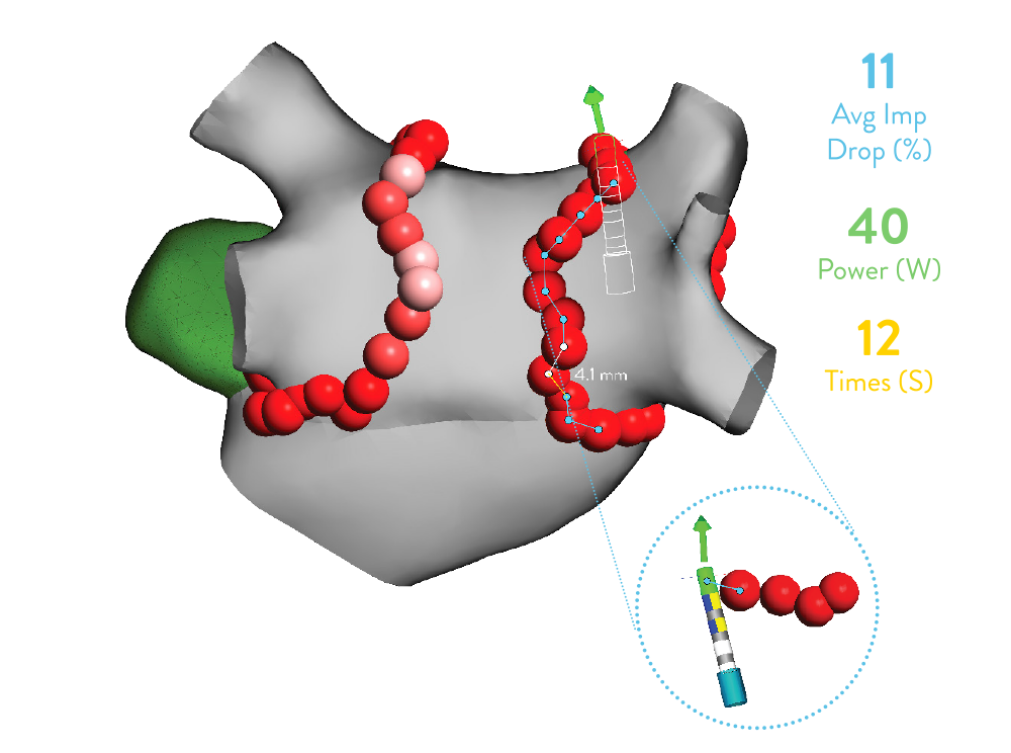
- Know the distance between AutoMarks for safe2,4 contiguous lesions. Gain objectivity by determining how far you’ve moved from your previous ablation point through AutoMark distance with the EnSite Aid Module.
- Enhance predictability for lesion transmurality by measuring Averaged Impedance Drop1.
EnSite™ VoXel Flex Mode
Reduced radiation with a low-fluoro workflow
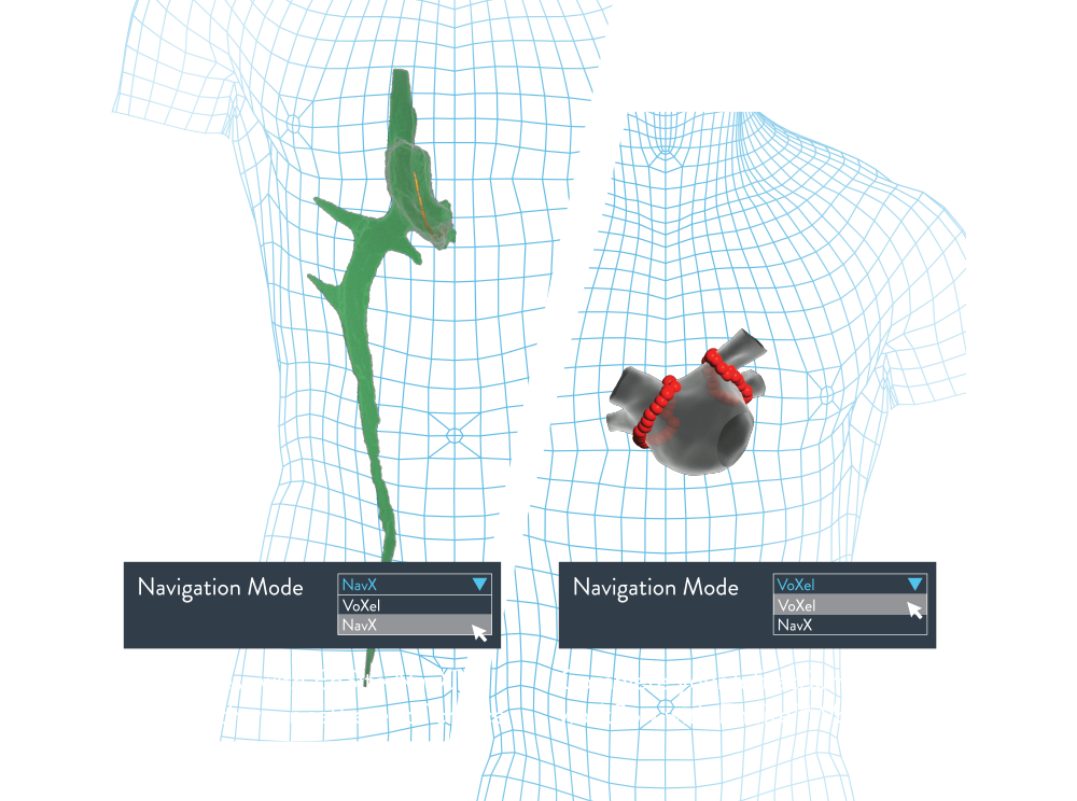
- Reduce radiation3 by seamlessly switching between impedance EnSite NavX™ Mode and magnetic EnSite™ VoXel Mode throughout the case.
- Experience the reliable stability of EnSite VoXel Mode in all procedures, when you want, with one click.
EnSite™ LiveSync Module
Changing the definition of software connectivity

- Integrate 3rd party systems (Stereotaxis‡ and Volta‡) with EnSite™ X EP System for a streamlined workflow.
- Increase workflow efficiency3 and tailor your case to each patient5.

Please complete the form below if you would like more information on the EnSite X EP System and a member of the Abbott EP team will be in touch.
Thank you for expressing your interest in learning more about how EnSite X Software Version 3 can optimize your workflow. Your rep or another member of our team will be reaching out shortly. You can find more information about the EnSite X EP System, a next-generation 3D mapping platform, by clicking the button below.
EnSite X EP System
- Friedman D et al. Impact of Filtered Impedance Drop on Atrial Lesion Size Prediction. Heart Rhythm. (In press)
- Abbott report 90997651 on file.
- https://pubmed.ncbi.nlm.nih.gov/28607610
- https://www.heartrhythmjournal.com/article/s1547-5271(23)00542-8/fulltext (Abstract on interlesion distance that just came out at HRS)
- https://pubmed.ncbi.nlm.nih.gov/28104073/ (Volta publication in which the conclusion explicitly states “patient-tailored’)
Rx Only. Brief Summary: Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events, and directions for use.
United States: Required Safety Information
Indications: The EnSite™ X EP System is a suggested diagnostic tool in patients for whom electrophysiology studies have been indicated. The EnSite™ X EP System provides information about the electrical activity of the heart and displays catheter location during conventional electrophysiological (EP) procedures. Warnings: For patient safety, any connections that directly connect the patient to the EnSite™ X EP System must be routed through the appropriate modules: EnSite™ X EP System SurfaceLink Module, EnSite™ X EP System 20 pin Catheter Input Module, EnSite™ X EP System 80-pin Catheter Input Module and Direct Connect Ports on the EnSite™ X EP System Amplifier. When using the EnSite™ X EP System, full protection against the effects of cardiac defibrillator discharge and other leakage currents is dependent upon the use of appropriate cables. The use of this device in conjunction with radio frequency ablation, as a part of the diagnosis and treatment of cardiac arrhythmias, may pose an increased risk of adverse events such as cardiac perforation, myocardial infarction, air embolism, and hematoma requiring surgical repair and/or blood transfusion. Non-SE catheters cannot collect location data and should not be used for navigation in VoXel Mode because they do not have a magnetic sensor. However, they can be visualized and display intracardiac signals. Only connect items that have been specified as part of the EnSite™ X EP System or compatible with the EnSite™ X EP System to the multiple socket-outlets. The EnSite™ X EP System model display should be used in conjunction with conventional EP techniques to confirm catheter location. The AutoMark feature does not indicate lesion effectiveness. AutoMarks are placed based on user-defined parameters for catheter stability and RF metrics only. Sudden impedance changes of the body or catheter electrodes caused by the connection of other devices (e.g., stimulator, defibrillator, and other devices) may create a location shift. Precautions: Ensure that surface electrodes, Patient Reference Sensors, and associated connectors do not contact one another, electrical ground, or metallic objects. EnSite™ X EP System components should be connected to power through an isolation transformer or the multiple socket outlet supplied with the system carts. Connecting equipment directly to a wall outlet may result in excessive leakage current. Do not operate the EnSite™ X EP System Field Frame within 10 m of another operating Field Frame. Do not place the EnSite™ X EP System Field Frame Cable inside the measurement volume or wrap it around the EnSite™ X EP System Field Frame, as it may create a magnetic interference. Metallic equipment used in close proximity to the magnetic field during the procedure, such as a sterile drape holder, may cause metal distortion. Do not place tool cables within 30 mm of the EnSite™ X EP System Field Frame Cable. If placed this close-particularly if the cables are parallel to each other the tool cable may become subject to electromagnetic interference. Do not use the EnSite™ X EP System in the presence of other magnetic fields. Do not drop the EnSite™ X EP System Field Frame or subject it to impact. Physical damage to the EnSite™ X EP System Field Frame may alter the EnSite™ X EP System Field Frame’s factory calibration.

Banner 7

Banner 8

Banner 9

Banner 10

Banner 11
